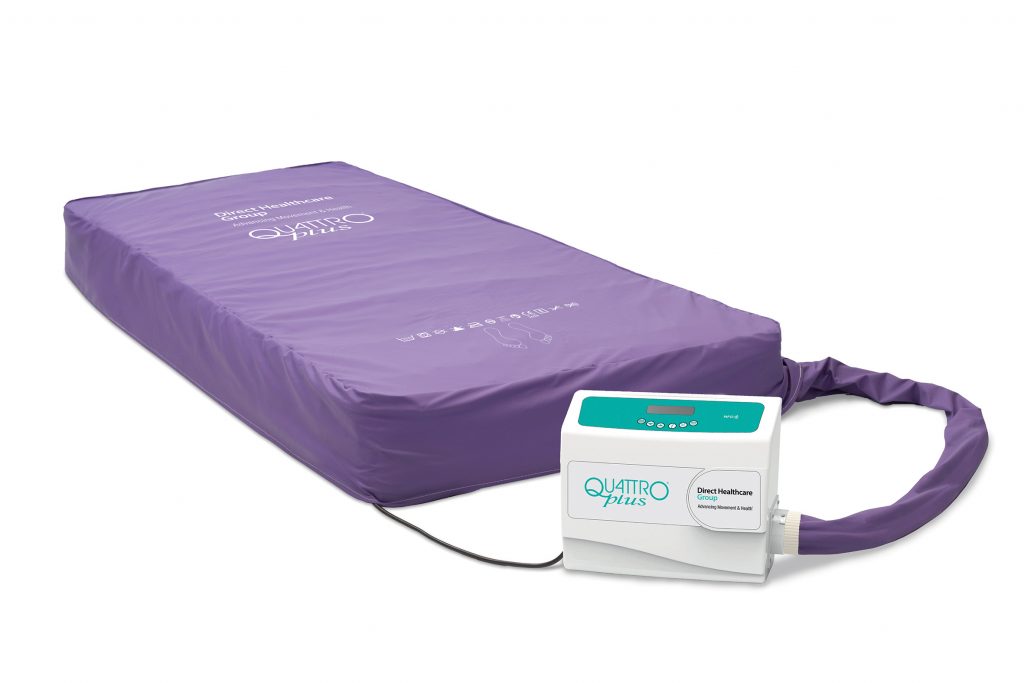
Quattro® Plus, the market leading and highly regarded 1-in-4 alternating therapy mattress, has been refreshed; combining the expertise of Talley Group and Direct Healthcare Group to bring a significantly overhauled control unit design and improved functionality that:
- Provides an easier to clean control unit, with reduced connection points, inserts and crevices, for adhesion to stricter infection control protocols.
- Clearer and easier operation of the control unit, with inputs and an LCD screen now located on the top panel.
- The addition of an NFC tag to enable simple reading of control unit information, whilst servicing information can be easily accessed through the system information button.
- Internal changes that make for easier servicing of cells.
- Improved CPR function for significantly reduced deflation times.
Suitable for all patient types, the Quattro® Plus mattress system combines an active 1-in-4 cell cycle, with TISSUEgard, Ortho-Differential Support and a multistretch cover to provide patients with pressure relief to prevent and manage patients at risk of or with existing pressure ulcers.
The active 1-in-4 cell cycle supports 75% of the patient’s body at all times, on groups of three inflated cells, whilst the fourth cell deflates sufficiently to redistribute the pressure and encourage tissue reperfusion. Additional benefits of the 1-in-4 system may include increased patient comfort, and less awareness of the support surface movement.
TISSUEgard™ enables the partial immersion and envelopment of the patient into the support surface, reducing the pressure on the patient’s skin and decreasing the pressure differential between inflated and deflated cells, helping to reduce associated shearing forces.
Ortho-Differential Support provides firmer outer edges of the mattress to facilitate patient transfers and provide extra support, whilst creating a softer central area of the mattress.